Clinical trials at CCMB
The treatments we use today are the result of previous clinical trials
Clinical trials are part of the cancer research continuum that builds on the work of fundamental laboratory and translational science by testing new advancements in patients that aim to improve all areas of cancer control. By enrolling in a clinical trial, patients help shape the future of cancer treatment.
Ask your physician if a trial exists for you.
How clinical trials benefit patients
You may receive a new beneficial treatment
Through clinical trials, patients may receive a cutting-edge treatment not widely available while also helping future cancer patients. Many of the cancer treatments patients receive today are the result of previous clinical trials.
CCMB, in partnership with patients, has participated in many studies over the years that directly improved the survival and quality of life of Manitobans suffering from cancer or blood disorders.
How clinical trials benefit cancer control
Future cancer patients may benefit from the findings of your trial
Since clinical trials are conducted primarily to improve patient outcomes over standard-of-care treatments, a major advantage of clinical trial participation is that all future patients benefit from the knowledge gained by past clinical trials.
Most oncologists are also clinical trialists. By fostering a robust Clinical Trials Unit, CCMB can competitively recruit the best and brightest physicians. Third party trial sponsors like drug companies help to fund made-in-Manitoba research advances.
Pedriatic Clinical Trials
Lorem ipsum odor amet, consectetuer adipiscing elit.
Lorem ipsum odor amet, consectetuer adipiscing elit. Ligula morbi litora odio phasellus leo aliquet potenti porttitor bibendum.
Inceptos nisi ullamcorper feugiat turpis nunc. Nisl nunc enim augue purus conubia quisque vestibulum odio dapibus. Penatibus ac taciti ornare leo magna?
Manitoba Prostrate Center
Lorem ipsum odor amet, consectetuer adipiscing elit.
Lorem ipsum odor amet, consectetuer adipiscing elit. Ligula morbi litora odio phasellus leo aliquet potenti porttitor bibendum.
Inceptos nisi ullamcorper feugiat turpis nunc. Nisl nunc enim augue purus conubia quisque vestibulum odio dapibus. Penatibus ac taciti ornare leo magna?
Manitoba Blood Marrow Transplant Program
Lorem ipsum odor amet, consectetuer adipiscing elit.
Lorem ipsum odor amet, consectetuer adipiscing elit. Ligula morbi litora odio phasellus leo aliquet potenti porttitor bibendum.
Inceptos nisi ullamcorper feugiat turpis nunc. Nisl nunc enim augue purus conubia quisque vestibulum odio dapibus. Penatibus ac taciti ornare leo magna?

Cancer research opens new options and better outcomes
Carole was diagnosed with lung cancer in 2020 shortly after retiring. The diagnosis came as a shock in no small part because Carole hadn’t smoked in over 40 years, and even then only socially.
Explore Patient Stories

May 16, 2024
Cancer research opens new options and better outcomes
Carole was diagnosed with lung cancer in 2020 shortly after retiring. The diagnosis came as a shock in no small part because Carole hadn’t smoked in over 40 years, and even then only socially.

May 16, 2024
Cancer research drives new treatment options
Shirley Mooney was first diagnosed with large B-cell lymphoma in 2008. She responded well to chemotherapy and, after six sessions, went into remission. Eleven years later, however, the cancer returned.
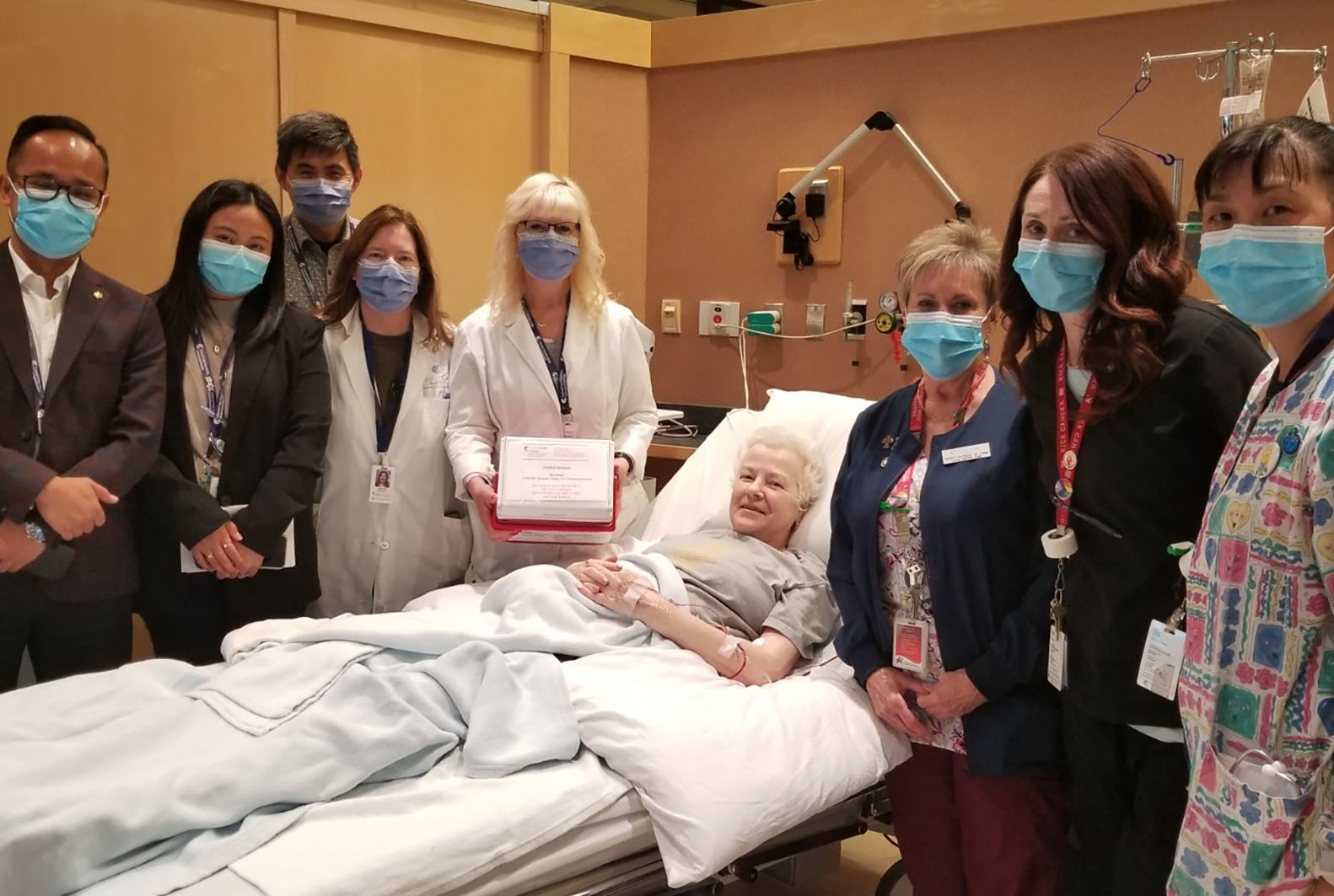
May 16, 2024
Leading-edge care close to home with a human touch
In 2014, Sandra was diagnosed with stage four lymphoma that manifested in her kidney. She never thought anything like cancer would happen to her.
What are clinical trials?
Research studies that involve people
Clinical trials test many types of cancer treatment such as new drugs, approaches to surgery or radiation therapy, or a combination. These studies, advanced from laboratory research, must be done with volunteer patients to determine whether the promising treatments are safe and effective.
Why are clinical trials important?
They help us understand the effectiveness of potential treatments
Clinical trials are essential for advancing cancer care. When a new treatment proves effective, it may become the new standard treatment that can help many patients. Progress made through clinical trials has resulted in thousands of people living longer with a better quality of life.
What is the Clinical Trials Unit at CCMB?
Most CCMB oncologists are also clinical trialists
The Clinical Trials Unit (CTU) facilitates and coordinates clinical research in the areas of cancer prevention, treatment, palliation, and quality of life. Our in-house oncologists, investigators, and support team enter about 300 new patients annually into about 120 adult and paediatric clinical trials.
Why does patient participation matter?
Clinical trials in patients are how new treatments are advanced
Patient participation is fundamental to clinical trials. The purpose of research is to evaluate the effectiveness of new care advancements in people where participants are always volunteers. Today’s cancer treatments have been made possible in part through past trial participants.
What are the advantages and disadvantages?
It’s important to become informed before you enroll
While there are rigorous protocols, safeguards, and oversight for all clinical trials, there are both advantages and potential disadvantages for patients to consider before enrolling. Part of the decision process includes in-depth communication between the patient and their oncologist. Learn more.
Enrolling your child in a clinical trial
Virtually all pediatric patients at CCMB are eligible for a clinical research study
It can be difficult for parents to decide whether to enroll their child in a clinical study. In addition to the potential of accessing a new beneficial treatment, some parents choose to participate to help us learn more about cancer in children to help future pediatric patients and their families. Pediatric trials require informed consent and have the same rigorous standards as adult trials.
How do I get started?
Ask your oncologist
The best way to learn if you or a loved one may be eligible for enrollment in a clinical trial at CCMB is to talk to your physician.
Contact us about Clinical Trials
In Manitoba: (204) 787-2197Toll-free: 1-866-561-1026
Email: [email protected]